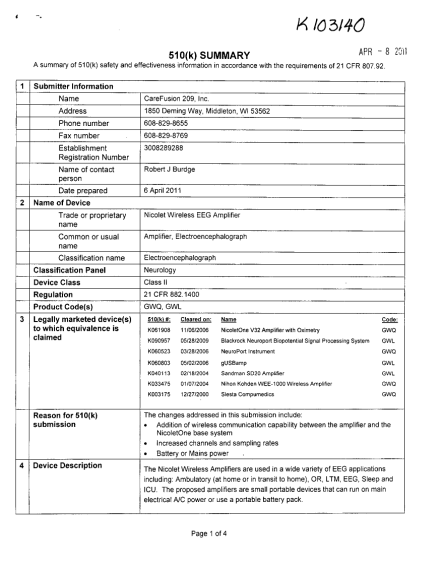
XK 16,3140 510(k) SUMMARY APR 8 20Q11 - A summary of 510(k) safety and effectiveness information in accordance with the requirements of 21 CFR 807,92 - accessdata fda
Xk 16,3140 510(k) summary apr 8 20q11 - a summary of 510(k) safety and effectiveness information in accordance with the requirements of 21 cfr 807,92. 1 submitter information name address phone number fax number establishment registration number...
FILL NOW