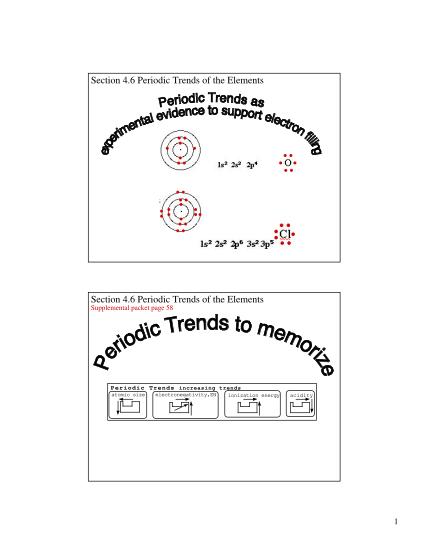
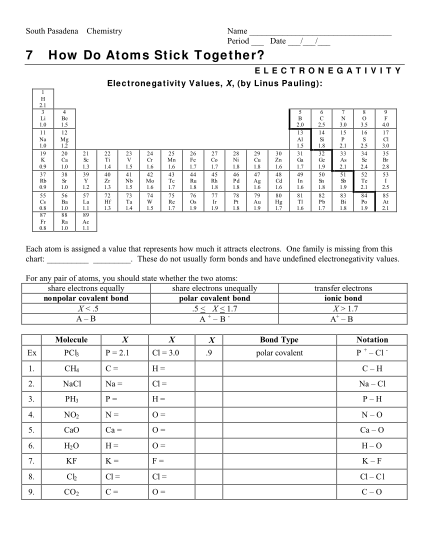
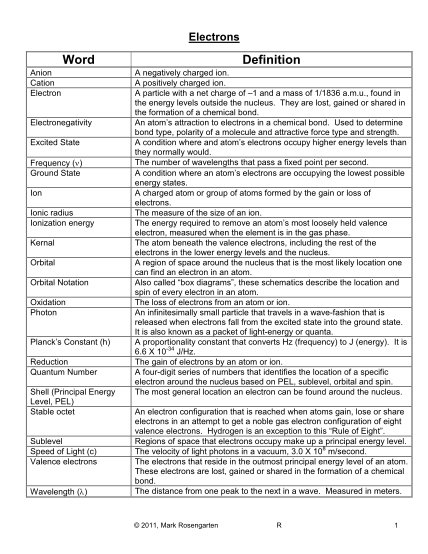
H. Buhl Johnston Heights Secondary School Chemistry 11
H. buhl johnston heights secondary school chemistry 11 texts: instructor: introduction: course outline. chemistry 11 evaluation & class expectation guide chemistry11. mcgrawhill ryerson., toronto, 2001 heath chemistry laboratory experiments, d.c....
FILL NOW