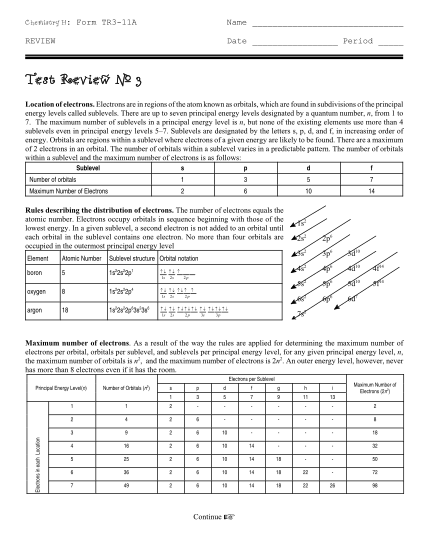
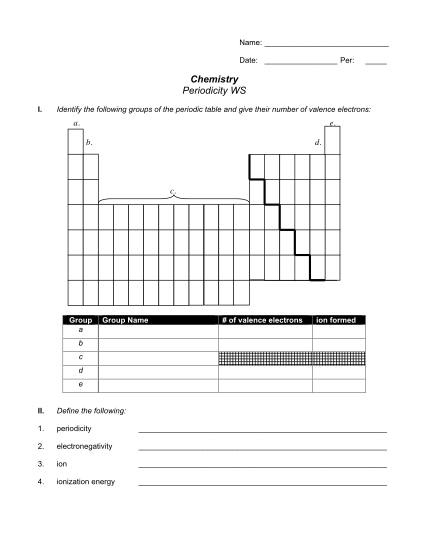
CESIUM - azmax co
Name mw bp c/mm (mp) d420 nd20 cesium atomic number crystal form 55 cs oxidation states bodycentered cubic atomic weight 0, 1 electrical resistivity (0c) 132.90543 electronegativity, pauling 19 cm cas number 0.7 enthalpy of melting 7440462...
FILL NOW